Uganda is making strides in the sector as it seeks to provide quality services to the citizenry. As part of efforts to achieve this, the government through the Ministry of Health has established three state-of-the-art Specialized Reference Laboratories under National Health Laboratory and Diagnostic Services (NHLDS).

They are; The Central Public Health Laboratories (CPHL), the National Tuberculosis Reference Laboratory (NTRL) and National Microbiology Reference Laboratory (NMRL), all of which are accredited to International Standards, meaning that their results are trusted anywhere in the world.

These reference Laboratories are supplemented by the Lab capacities in research institutes such as the Uganda Virus Research Institute (UVRI) Laboratory, Joint Clinical Research Centre (JCRC) and universities (Makerere, Mbarara, Gulu, etc) whenever need arises.
Major Capacities in the Reference Laboratories
The major Capacities of the National Health Laboratory Services are as follows: –
✓ Capacity to test for COVID-19 in Butabika, and with mobile Laboratories currently placed in Tororo, Adjumani Hospital (for Elegu border crossing), Mutukula and Mubende Regional Referral Hospital. Additionally, all the Regional Referral Hospitals have been equipped with GeneXpert machines which the department activates any time additional testing capacity is needed. These Laboratory has been the cornerstone in the Ministry’s strong response in the prevention, management and control of the ongoing COVID-19 by providing quality and real-time results for decision-making.

✓ Capacity of National TB Reference Laboratory (NTRL) to consistently provide surveillance and early detection of Multidrug Resistant Tuberculosis (MDR-TB) and Extremely Resistant TB (XDR-TB) to help in early detection and isolation of cases to prevent spread and protect life.
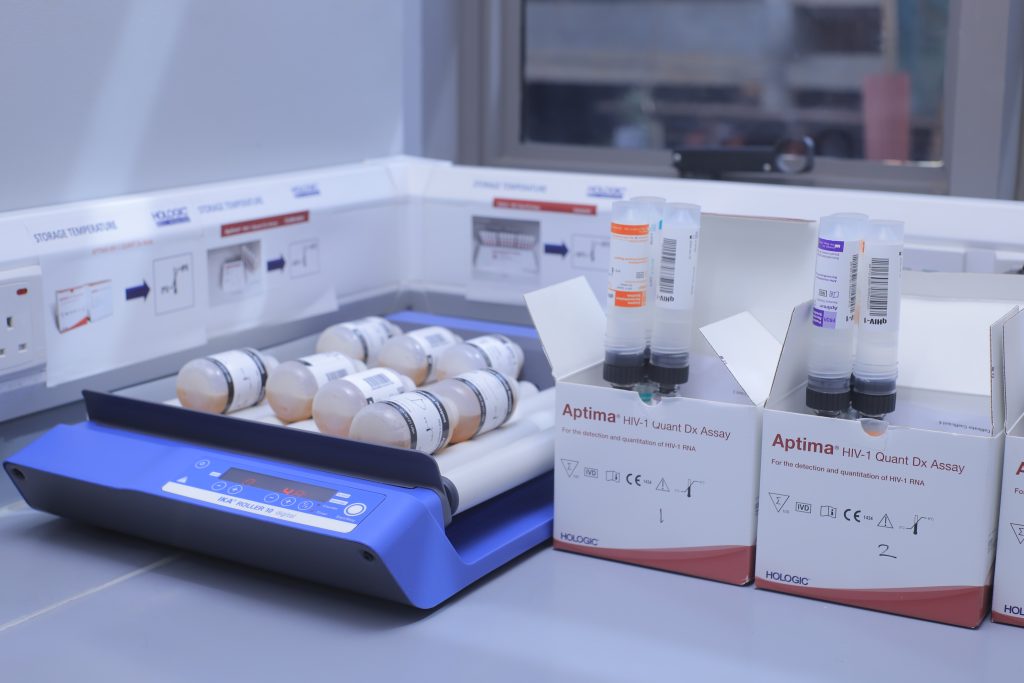
✓ Capacity to provide testing for diagnosis of HIV and Hepatitis B including Viral Load for up to 2.5 million tests every year thereby providing access door to life saving ARVs and treatment monitoring for people living with HIV/AIDS and Hepatitis B including babies and pregnant mothers.
✓ Capacity for sickle cell disease confirmation, Heme Path Cancer Diagnosis and other non-communicable and lifestyle disease diagnosis.
✓ Capacity for routine diagnostic check-ups for functions of the Kidney, Liver, Heart and other vital organs of the body.

✓ Established the genomic Laboratories at the Central Public Health Laboratory (CPHL) and National Tuberculosis Reference Laboratory (NTRL) for routine surveillance of any genetic changes in disease–causing microorganisms that may result in being very dangerous (like resistance to treatments) or weaker and therefore milder.
✓ Capacity of the National Microbiology Reference Laboratory to carry out routine bacterial culture and sensitivity for Antimicrobial Resistance (AMR) surveillance.

✓ Developed laboratory information management systems which is used at the in the refence Laboratories (CPHL and NTRL) to track patients’ samples and relay results to the Doctors. For example, the TB Lab information system tracks patient samples and rely results to MDR treatment sites in the country, which has greatly improved turn round time for TB culture and drug susceptibility results.
✓ The External Quality Assessment (EQA) Laboratory. The reference Laboratories at NHLDS produce Proficiency testing panels that are accredited to ISO Standards which are used to expertly control the quality of Laboratory diagnosis across the national health laboratory network.

✓ Equipment Calibration Centre. The NHLDS has an internationally accredited equipment calibration Centre that supports calibration and standardization of equipment in the whole Laboratory sub-sector in the country to ensure they provide accurate results.
✓ The Laboratory Department (through NTRL and CPHL) Supports 26 countries in Africa as part of the World Health Organization – given mandate, and has supported ISO 15189 accreditation of 11 National Reference laboratories in the region. This is marketing Uganda’s healthcare system internationally.


























